
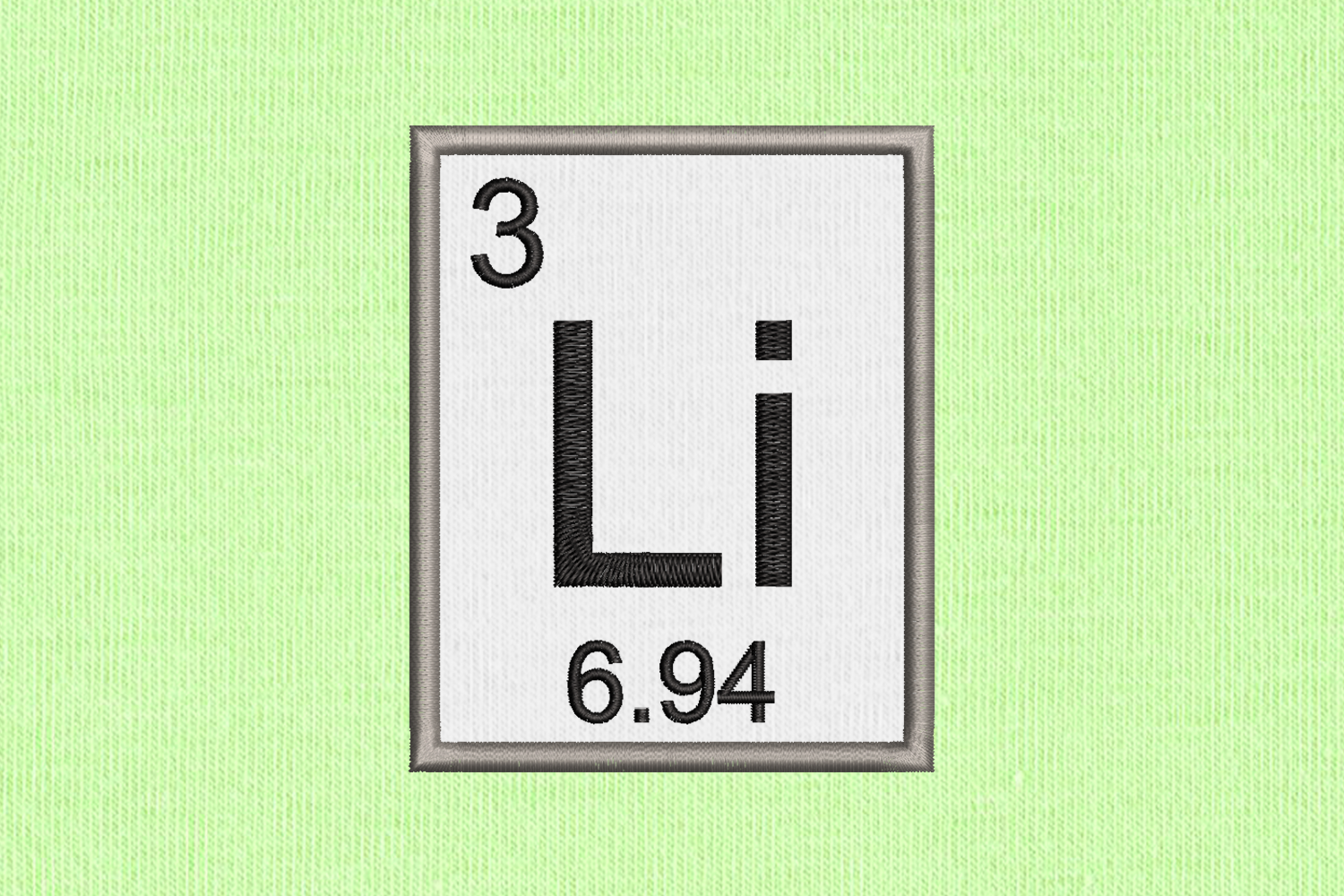
Because of its relative nuclear instability, lithium is less common in the solar system than 25 of the first 32 chemical elements even though its nuclei are very light: it is an exception to the trend that heavier nuclei are less common. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes found in nature have among the lowest binding energies per nucleon of all stable nuclides. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. Under standard conditions, it is the least dense metal and the least dense solid element. It is a soft, silvery-white alkali metal. Lithium (from Greek: λίθος, romanized: lithos, lit.'stone') is a chemical element with the symbol Li and atomic number 3.


 0 kommentar(er)
0 kommentar(er)
